Transcriptomic Profiling of Plaque Psoriasis and Cutaneous T-Cell Subsets during Treatment with Secukinumab - ScienceDirect
Cosentyx® Novartis Biociências SA Solução injetável 150 mg/mL Contém: 1 ou 2 canetas preenchidas Bula do Paciente

Transcriptomic Profiling of Plaque Psoriasis and Cutaneous T-Cell Subsets during Treatment with Secukinumab - ScienceDirect

COSENTYX 150 MG INJEKČNÝ ROZTOK V NAPLNENEJ INJEKČNEJ STRIEKAČKE sol inj 1x1 ml/150 mg (striek.inj.skl.) - Príbalový leták

PDF) Repurposing of Secukinumab as Neuroprotective in Cuprizone-Induced Multiple Sclerosis Experimental Model via Inhibition of Oxidative, Inflammatory, and Neurodegenerative Signaling

Transcriptomic Profiling of Plaque Psoriasis and Cutaneous T-Cell Subsets during Treatment with Secukinumab - ScienceDirect
COSENTYX 150 MG INJEKČNÝ ROZTOK V NAPLNENEJ INJEKČNEJ STRIEKAČKE sol inj 1x1 ml/150 mg (striek.inj.skl.) - Príbalový leták

COSENTYX 150 MG INJEKČNÝ ROZTOK V NAPLNENEJ INJEKČNEJ STRIEKAČKE sol inj 1x1 ml/150 mg (striek.inj.skl.) - Príbalový leták
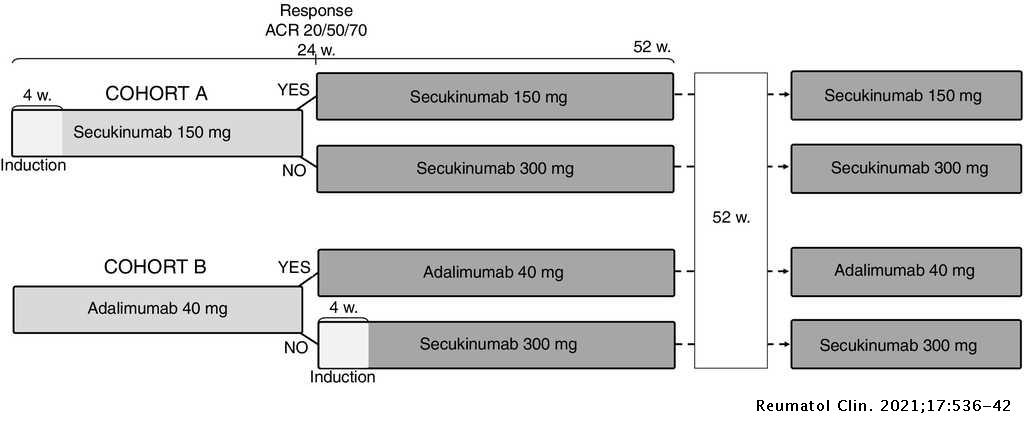
A cost-consequence analysis of the preferential use of secukinumab versus adalimumab for the treatment of psoriatic arthritis | Reumatología Clínica
COSENTYX 150 MG INJEKČNÝ ROZTOK V NAPLNENEJ INJEKČNEJ STRIEKAČKE sol inj 6(3x2)x1 ml/150 mg (striek.inj.skl.) - Príbalový leták
COSENTYX 150 MG INJEKČNÝ ROZTOK V NAPLNENEJ INJEKČNEJ STRIEKAČKE sol inj 1x1 ml/150 mg (striek.inj.skl.) - Príbalový leták
PT. Pfizer Indonesia Local Product Document Product Document Title: Recombinant Somatropin Trade Name: Genotropin GoQuick CDS Ef

Cosentyx Novartis. Mi experiencia. - Hoy toca vacunarse! La verdad estas vacunas son una maravilla. A penas tengo un poco de brote en la cabeza pero el resto del cuerpo perfecto. | Facebook
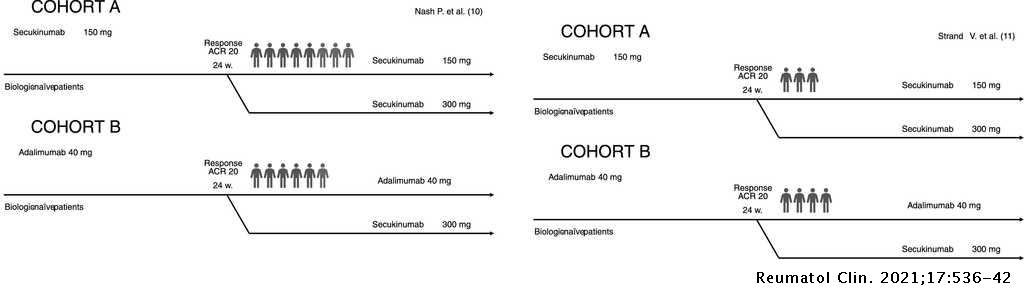
A cost-consequence analysis of the preferential use of secukinumab versus adalimumab for the treatment of psoriatic arthritis | Reumatología Clínica
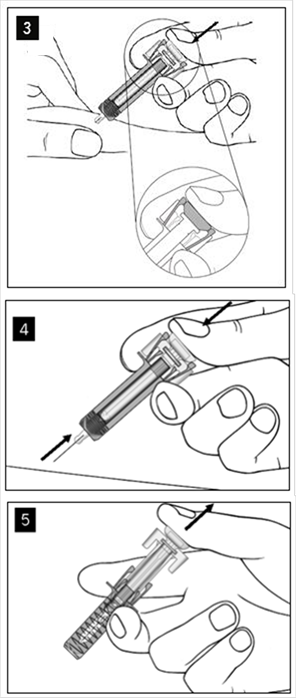
COSENTYX 150 MG INJEKČNÝ ROZTOK V NAPLNENEJ INJEKČNEJ STRIEKAČKE sol inj 1x1 ml/150 mg (striek.inj.skl.) - Príbalový leták