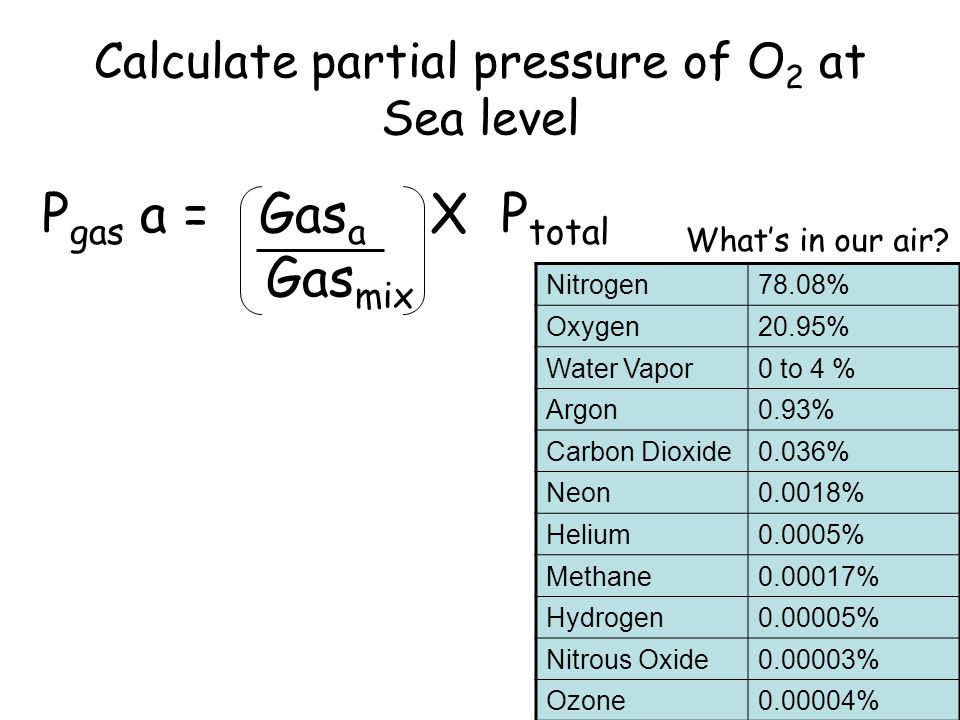
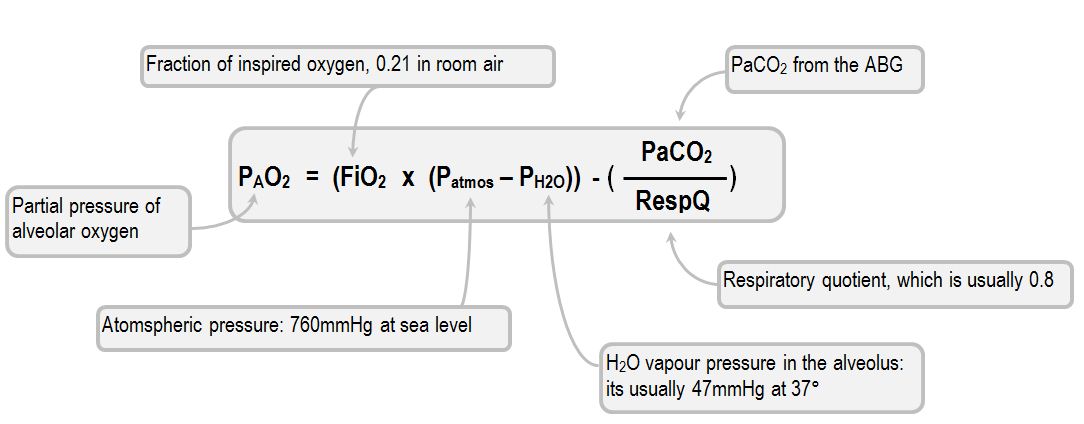
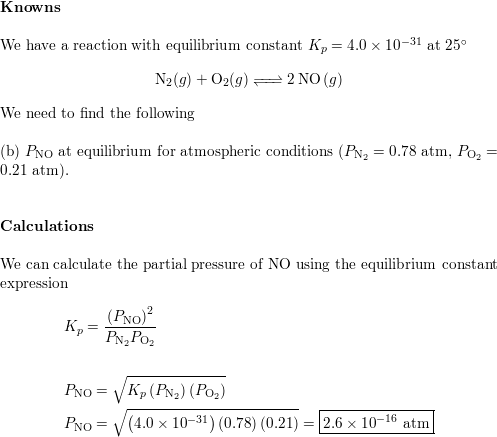
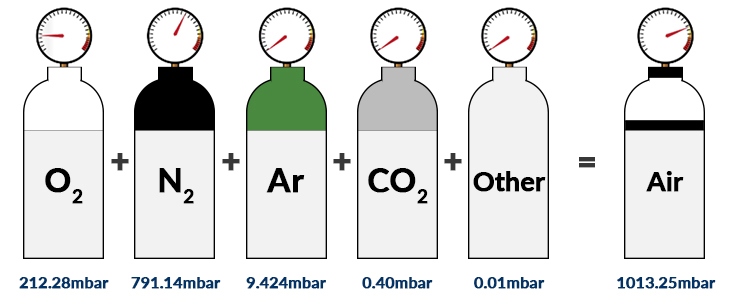
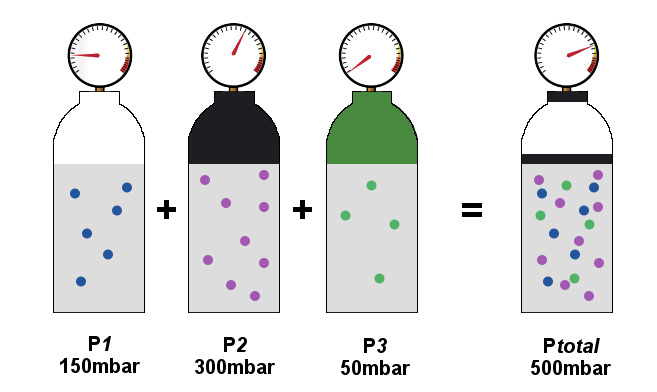
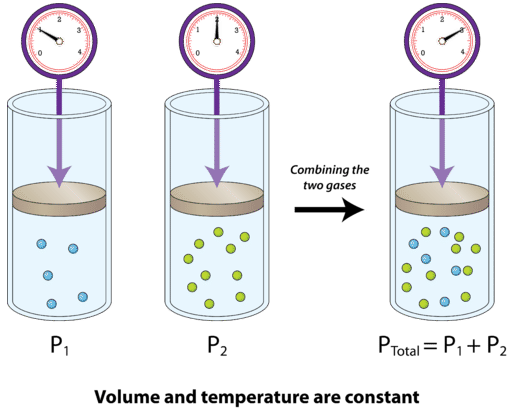
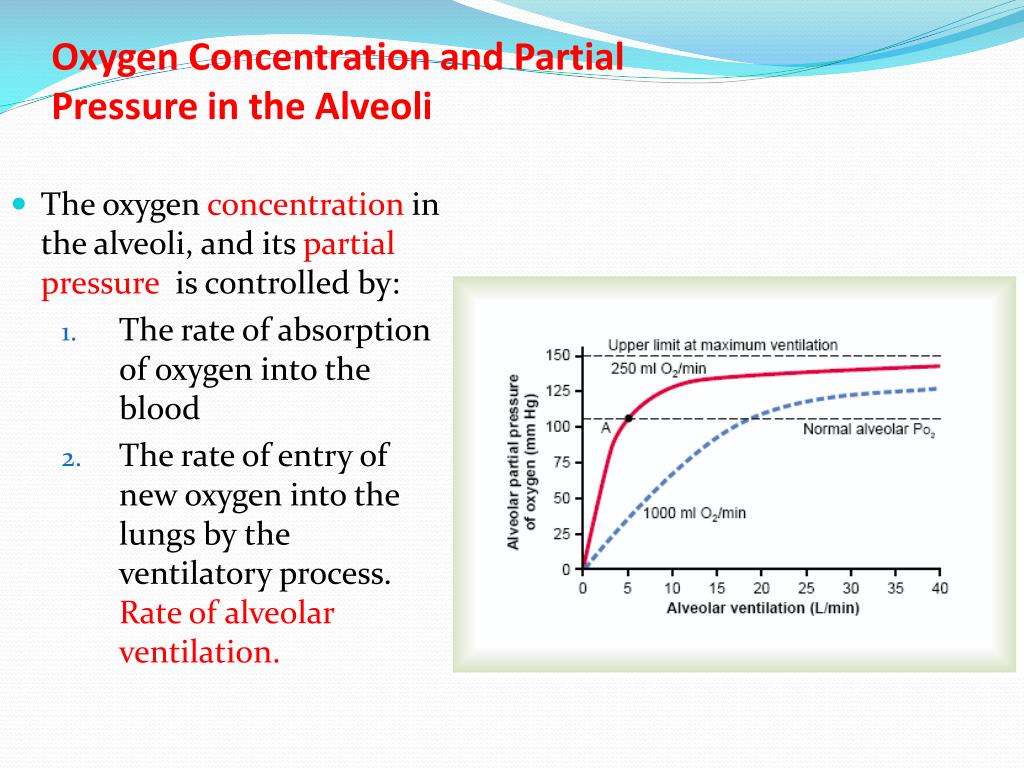
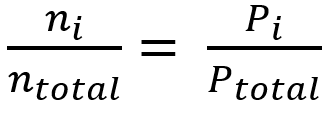SOLVED: Calculate the partial pressure of each of the following gases in room. Air when the barometric pressure is 760 mmHg ( assume the room air contains 21% oxygen, 78% nitrogen, and
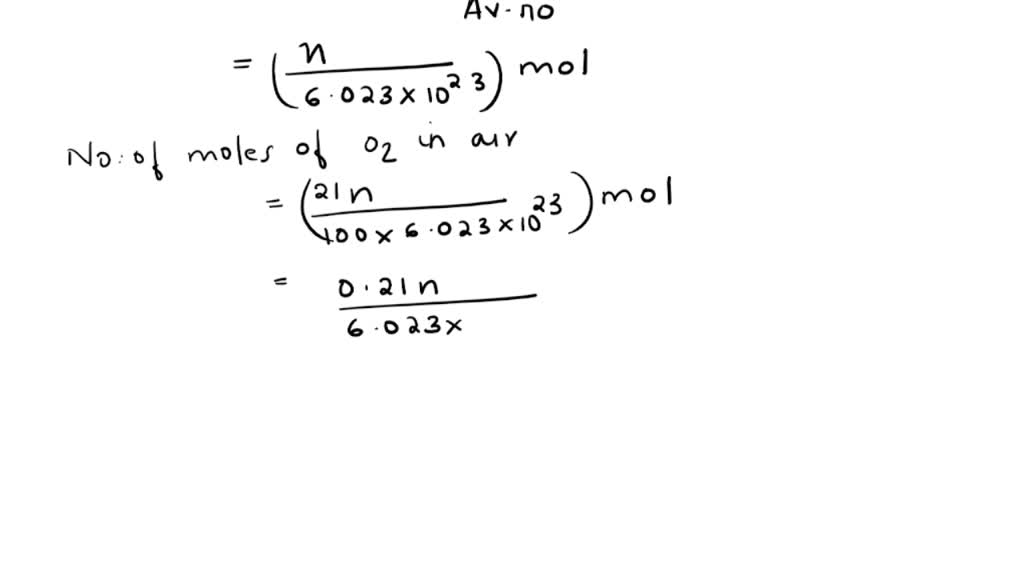
SOLVED: calculate the partial pressure of O2 (in atm) in dry air when the atmospheric pressure is 754 mmHg.