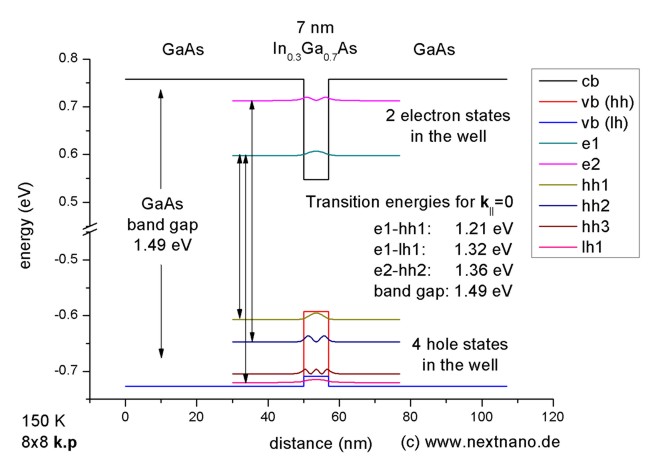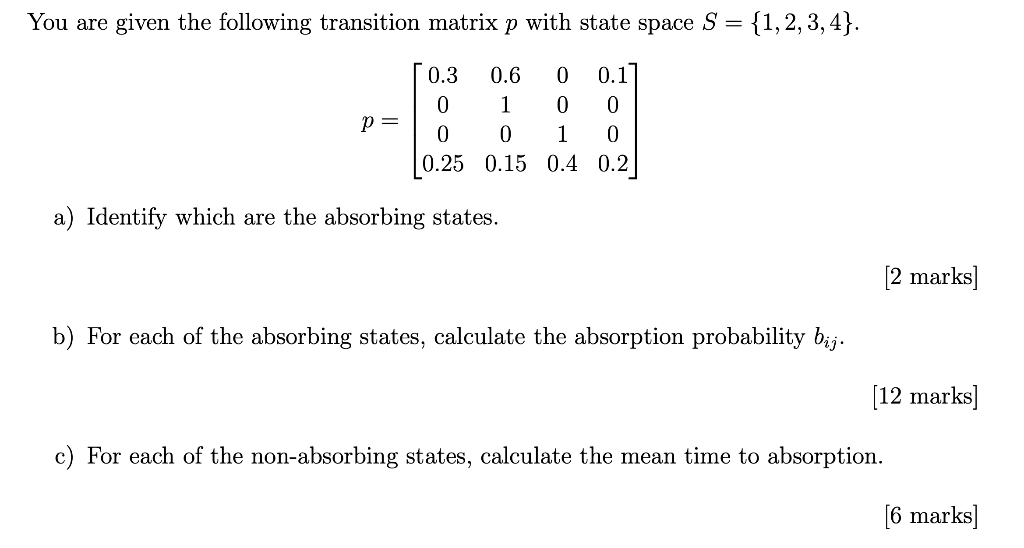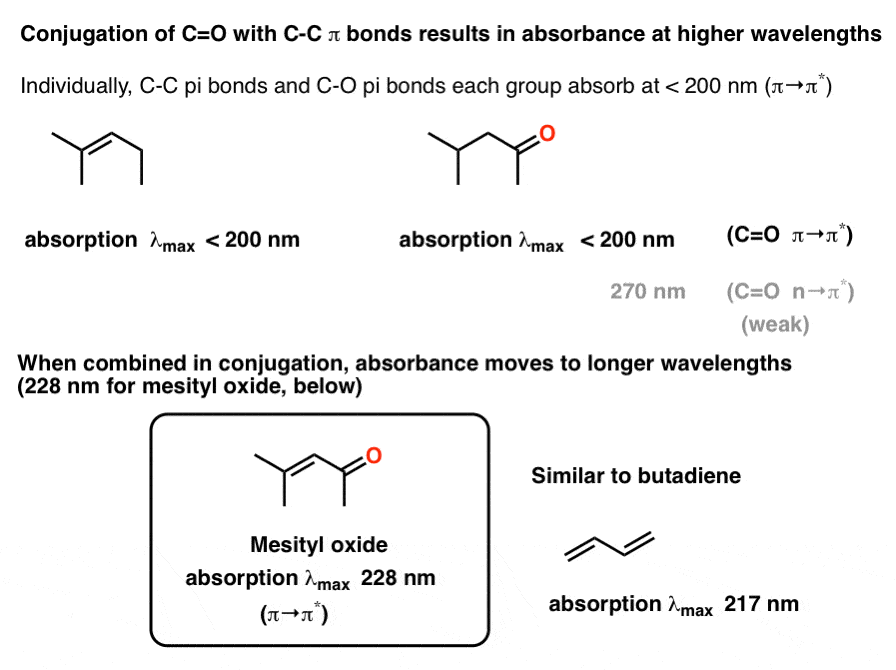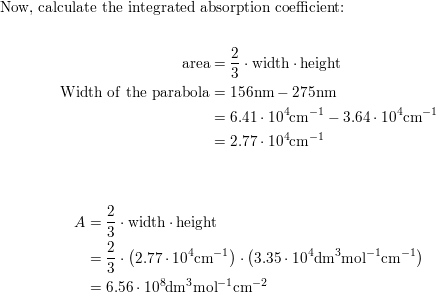
The longest wavelength doublet absorption transition is observed at 589.0 ans 589.6nm . Calculate the frequency of each transition and energy difference between two excited states .

Transition cross-sections, explained by RP Photonics Encyclopedia; laser, emission, absorption cross-sections, gain coefficient

Femtosecond Absorption Spectroscopy of Transition Metal Charge-Transfer Complexes | Accounts of Chemical Research

First-principles modelling of the L-edge X-Ray Absorption Spectroscopy of Transition Metal Oxides and Organic Molecules with Transition Metals Centers – eSSENCE
CALCULATION OF THE TRANSITION DIPOLE MOMENT OF THE $\tilde{A}\leftarrow \tilde{X}$ ELECTRONIC TRANSITION OF THE C$_2$H$_5$O$_2$ FROM THE PEAK ABSORPTION CROSS-SECTION

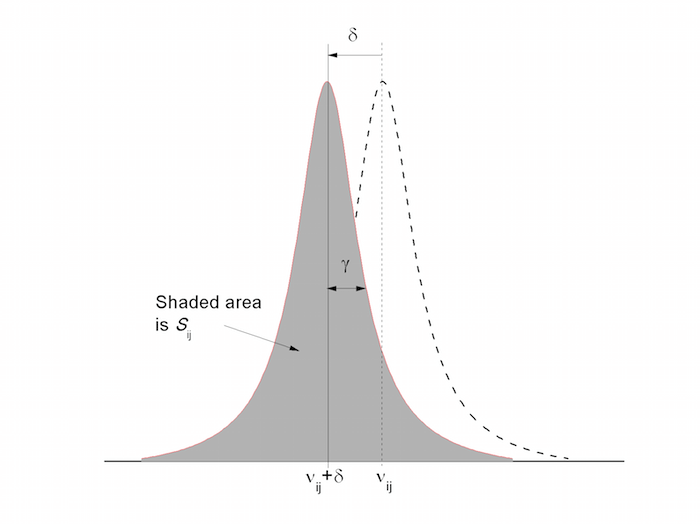
In a one-body picture, there is only one transition in the absorption... | Download Scientific Diagram
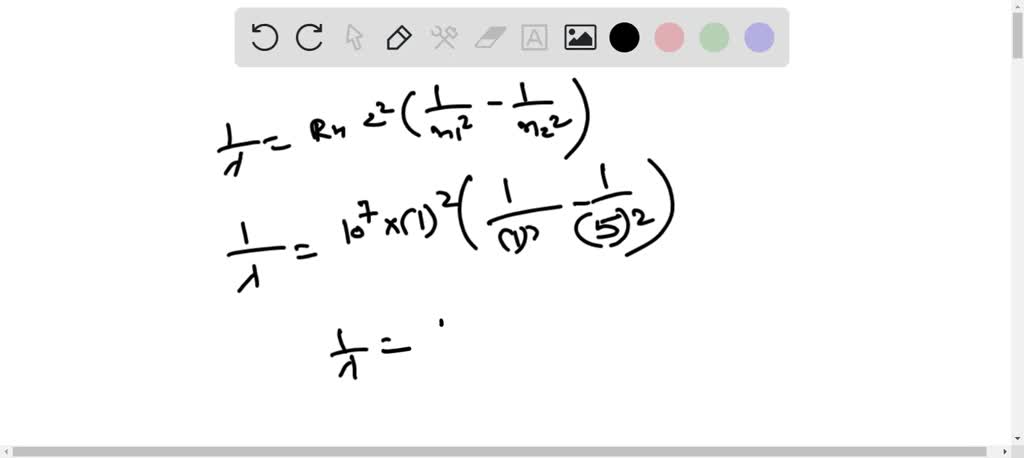
SOLVED: Calculate the wavelength of the line in the absorption line spectrum of hydrogen caused by the transition of the electron from an orbital with n=1 to an orbital with n=5. Round

Calculate the wavelength of the line in the absorption line spectrum of hydrogen caused by the transition of the

Calculated absorption spectra from the transition oscillator strength.... | Download Scientific Diagram

CHEM1011 notes for 8 topics - Atomic spectra & Rydberg 1. Relate the lines and series in the - Studocu

SOLVED: 2) Which of these transitions correspond to emission and which to absorption? a. n = 2 to n = 4, Absorption b n = 3to n = 1, Emission C. n =
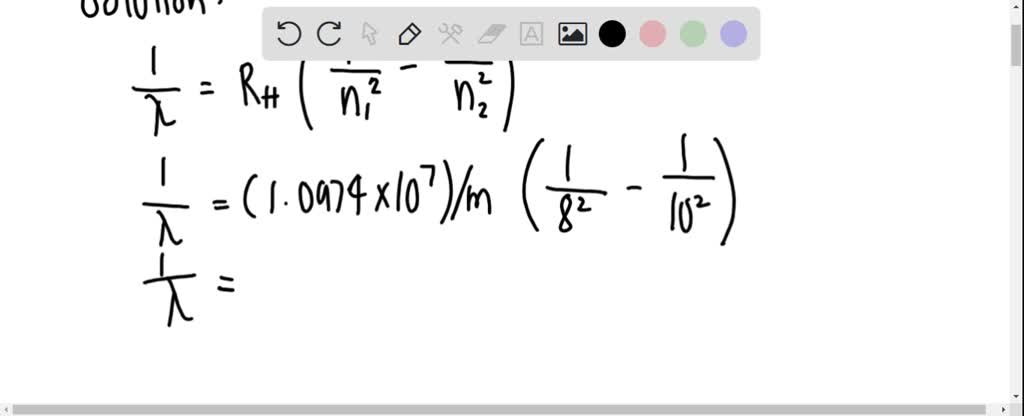
SOLVED: Calculate the wavelength of the line in the absorption line spectrum of hydrogen caused by the transition of the electron from an orbital with n=8 to an orbital with n=10. Round
CALCULATION OF THE TRANSITION DIPOLE MOMENT OF THE $\tilde{A}\leftarrow \tilde{X}$ ELECTRONIC TRANSITION OF THE C$_2$H$_5$O$_2$ FROM THE PEAK ABSORPTION CROSS-SECTION